Exploring Free Energy Surfaces with MACE-PLUMED Metadynamics
In this tutorial, we will learn how to link up the recently developed MACE potential with PLUMED to perform enhanced sampling simulations in the form of two-dimensional metadynamics. Our test system will be a molecule of gaseous carbonic acid (H2CO3). This tutorial will probe the various conformations carbonic acid can adopt and establish which are the most stable.
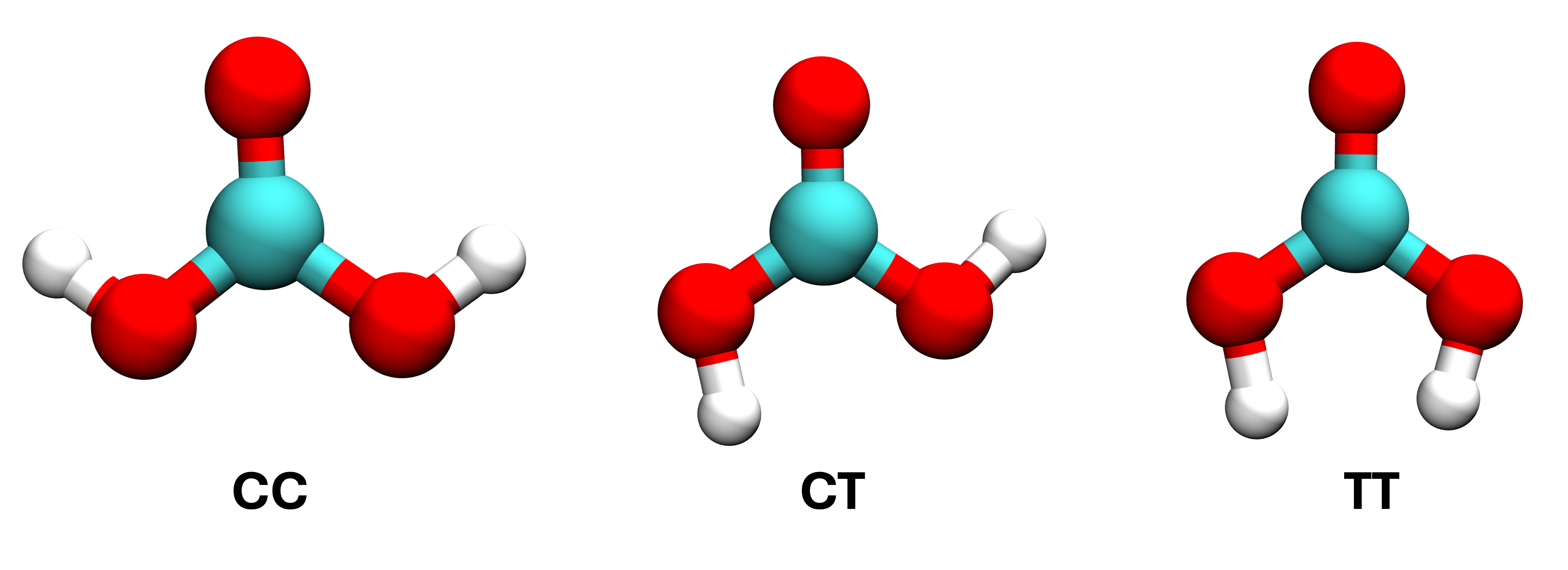
Carbonic acid is important for carbon dioxide solvation chemistry, relating to processes like ocean acidification and the bicarbonate buffer system. The acid has two terminal OH groups that can either be in cis or trans orientation, giving rise to three conformers, cis-cis, cis-trans, and trans-trans as shown below.
In this tutorial, we will cover:
- Navigating the MACE-LAMMPS-PLUMED interface.
- Setting up your work environment.
- Running simple MD simulations using a MACE potential.
- Performing metadynamics using two collective variables.
By the end of this tutorial you should:
- Know how to run simple MD powered by MACE potentials.
- Integrate MACE with PLUMED to enable the accurate determination of free energy surfaces.
The data and files needed to run the excercises of this tutorial can be found on Github. Clone the following directory to your local machine: https://github.com/water-ice-group/plumed_tutorial_mace.git
This tutorial is organized as follows:
flowchart TD
PRE[Metadynamics] ==> B[Setting up your environment]
A[Introduction to MACE] ==> B[Setting up your environment];
B ==> C[Running Free MD]
C ==> D[Running Metadynamics];
click PRE "../../../21/004/data/NAVIGATION.html" "Masterclass on Metadynamics"
click A "01_mace_intro.html" "Learn about the MACE potential for performing accurate and efficient molecular dynamics simulations"
click B "02_setting_up_env.html" "Understand how to set up the MACE-ASE-PLUMED interface."
click C "03_freeMD.html" "Run simple MD using MACE and ASE."
click D "04_metaD.html" "Perform metadynamics using MACE to examine conformational stability."
Click here to open manual pages for actions discussed in this tutorial.
